Why do metals corrode? How it is prevented?

Written By Dr. Fatima Saifee
March 17, 2021
Corrosion

What is Corrosion of metals?
Corrosion is a naturally occurring process an unwelcome occurrence, caused by the dissolution of metal through an accidental, chemical, or electrochemical reaction at the boundary of metal and aqueous medium. Corrosion term originates from the Latin word ‘corrode which means eat away. Corrosion is regarded as the cancer of metals that arises from thermodynamic instability.
There is a different statement of corrosion by several writers, philosophers, and scientists that achievement of any scientific review depends upon accurate and definite descriptions of the phenomena being studied.
The corrosion process is known by its physical nature of metal loss with its environment and the mechanism, how it corrodes the metal. It can be physical damage to the metal concerning its environment in which it takes place.
Corrosion is an important phenomenon to differentiate between the form of damage, the mechanism by which the material damage, and the environment which supported the mechanism.
Definition of Corrosion
Corrosion can be defined in many ways-
Corrosion is simply defined as the degradation of materials or any alloy by chemical or electrochemical reaction and the aggressive electrolyte.
Corrosion can be defined as “the spontaneous process of degradation and deterioration or destruction of metallic construction in the course of their chemical, biochemical or electrochemical interactions with the surroundings”.
Corrosion can also be defined as a deterioration of a material, for example, a metal like iron, copper, etc. with its environment by an electrochemical process.
Corrosion is one of the most interesting fields of electrochemistry. Corrosion of metals is based on thermodynamics, it is the science of energy flow. Metals seldom exist in nature as pure metals. Metals oxidized more easily than others when they absorb energy and become more stable form by the process of corrosion. Normally metals are thermodynamically unstable and occur in nature most commonly as oxides, carbonates, sulfides, chlorides, and silicates. Pure metal extracted from ore is in a higher energy state than their ores with a lower energy state which is an oxide or some other compound. Metal is extracted from ore through metallurgical processes, involves a reduction of the oxidized form to free metal which requires a significant amount of energy. Thus all metals tend to revert to more stable compounds, one of nature’s fundamental reactions is reversed. This process of decaying through a chemical or electrochemical reaction with the environment is known as corrosion. Few metals do not corrode e.g. noble metals such as Gold and Platinum are resistant to corrosion. Although Corrosion of metals is unavoidable can thus never be stopped completely, but can be significantly controlled. It is observed that all environments are corrosive to some grade. Corrosion can be slow or fast depending on the environment to which it is exposed. Most industries are affected by corrosion such as petroleum, aircraft, home appliances, pharmaceutical, telecommunications, etc.
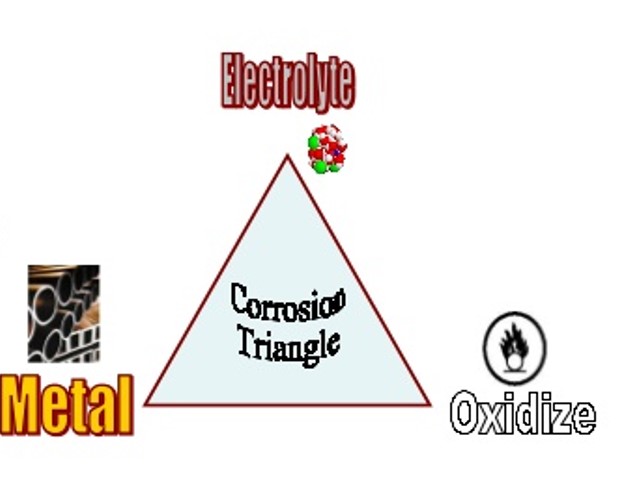
How corrosion occurs?
Corrosion occurs at the interface between a metal and an aqueous solution by an electrochemical process.
To start the corrosion, an oxidation reaction and a reduction reaction must occur simultaneously. A thin condensed film of moisture is enough to proceed with the corrosion reaction.
Corrosion Mechanisms
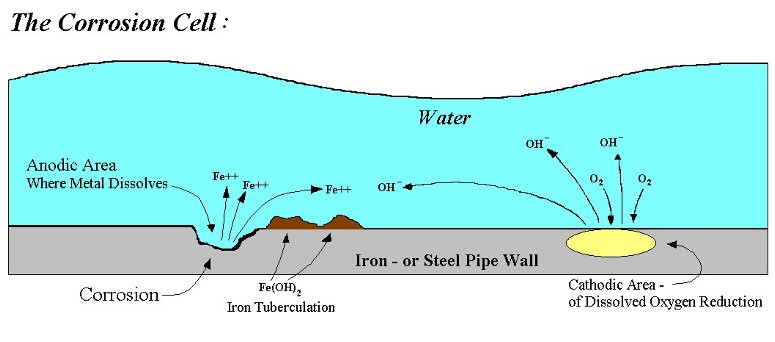
Corrosion is a fundamental process, when metal reacts with its environment which involves the transfer of electrons as a result of electrochemical reaction, depends on both the metal and the environment to which it is exposed. It is essential to recognize the fundamentals of corrosion to identifying the mechanism of corrosion as well as for predicting the corrosion behavior of metals in an aqueous environment. Corrosion reaction occurs at the edge of a metal and an electrolyte. Corrosion of metals in an oxygenated aqueous environment involves the movement of electrons between the metal and the electrolyte (e.g. water).
A basic corrosion cell consists of 4 elements
- Anode
- Cathode
- A metallic electrical connection between the anode and cathode (e.g. the pipe wall)
- Electrolyte (Corrosive medium).
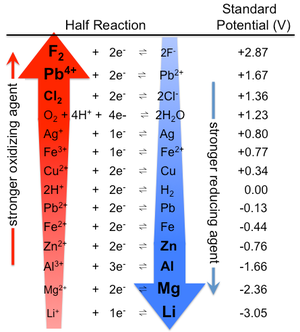
What Factors affecting the corrosion of metals?
Corrosion generally depends on the nature of the metallic material and the corroding medium. The rate, extent, and type of corrosion depend on the surrounding in which the metal is to be unprotected, the concentration of corrosive media, and the nature of chemical properties of metal or alloys which influence the aqueous corrosion. Factors that are responsible for corrosion are the nature of metal purity and position of the metal in galvanic series, electrode potential difference, hydrogen overvoltage, the composition of the metal, solubility in corrosive media, and nature of film formed on the surface. Corrosion is also influenced by secondary factors like temperature, pH of the solution, conductivity, the amount of dissolved oxygen, the presence of trace amounts of impurities, the degree of agitation, wet chemistry, flow velocity, pressure, heat treatment, presence or absence of an inhibitor, Microbial growth, ratio of oil/water and state of an inner surface of the steel. Any changes in these factors can alter in corrosion rate significantly. The rate of corrosion is governed by the activation energy required for the corrosion process or electrochemical reaction (oxidation and reduction) and the mechanism of occurring corrosion. Metallic corrosion is a vibrant process and subject to arbitrary variations even under constant conditions.
Methods of Corrosion Prevention and Control
Corrosion protection is constant, dynamic process. Controlling corrosion effectively can increase the life of all equipment, water, oil, and gas pipelines. Corrosion control techniques are used to prevent corrosion. Proper maintenance, effective design of equipment, use of the latest technology should be monitoring by qualified specialists. There are many methods to mitigate corrosion are materials selection, Corrosion Inhibitors, Scale inhibitors, Biocide Treatment, Oxygen Scavenger, H2S Scavenger, Cathodic protection, Pigging, protective coatings, and linings, Regular Monitoring/Inspection.

thanks, interesting read
Thanks, I’ve been looking for this for a long time
very good